Abstract
The molecular mechanisms underlying lineage commitment of stem and progenitor cells have implications for deriving specific cell types in vitro for regenerative medicine purposes. Current approaches to derive transfusable amounts of erythrocytes and platelets fall short of producing physiologically relevant amounts of each cell type. Thrombopoietin (TPO), commonly used in culture systems to increase platelet yield from cultured megakaryocytes, increases megakaryocyte (Mk) numbers and promotes platelet production. With the goal of generating adequate quantities of transfusion products in mind, establishing differentiation cultures that start with highly purified progenitors with Mk and E lineage potential as well as expansion potential is a very attractive option. However, a pervasive misconception exists that supplementing MEP cultures with TPO will increase the frequency with which the MEP produce megakaryocyte-committed daughter cells, thereby increasing the pool of lineage-restricted progenitor cells with high expansion capacity that ultimately yield higher numbers of transfusable platelets. We investigated whether TPO plays a role in instructing Megakaryocytic-Erythroid Progenitor (MEP) [1] commitment to the Mk lineage utilizing our previously presented time-lapse imaged colony forming unit assay and cell tracking approach [2]. This allowed us to calculate the frequency with which MEP give rise to lineage restricted progenitors in control conditions (with SCF, IL3, IL6, EPO, and TPO) compared to conditions lacking TPO to test the hypothesis that absence of TPO may diminish the frequency with which MEP give rise to Mk-restricted progenitors.
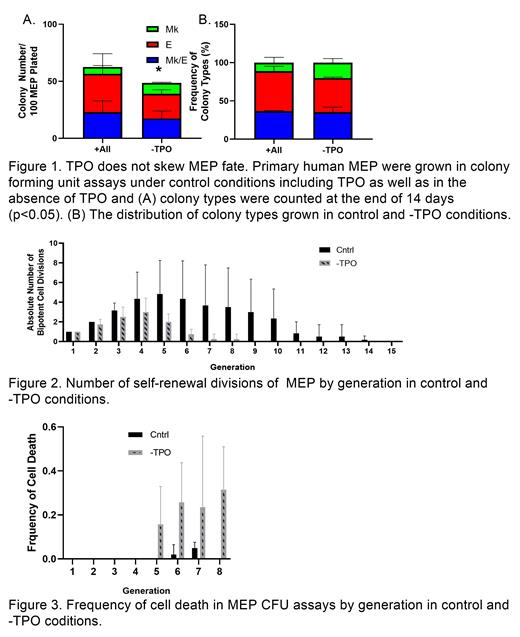
We found that in the absence of TPO, MEP colony forming efficiency significantly (p<0.05) dropped by 22.4%. Importantly, the distribution of colony types was unaffected by the absence of TPO (Figure 1). By time-lapse imaging we observed the frequency with which MEP commit to the E lineage versus the Mk lineage to be approximately 2:1 in both control and -TPO conditions, demonstrating that TPO does not affect MEP lineage commitment
Time-lapse imaging also confirmed that MEP cultured without TPO underwent significantly (p<0.05) fewer symmetric self-renewal divisions resulting in less expansion of MEP, as well as less asymmetric self-renewal divisions resulting in more rapid exhaustion of MEP (Figure 2). 80% of control MEP divisions resulted in self renewal for an average of 8 generations and expansion divisions dropped below 10% after the 6 th generation, whereas MEP cultured without TPO lost the same propensity to self-renew within 3 generations. This confirms that TPO supports MEP self-renewal and expansion, similarly to how TPO supports HSC expansion [3].
Cell death rates were at least 5-fold higher in the absence of TPO compared to control culture conditions containing TPO, and this effect was observed in both E and Mk-destined cells, demonstrating that TPO supports survival of both E and Mk-destined progenitors (p<0.05; Figure 3). Of note, CD41 mean fluorescence intensity was 2-fold dimmer in Mk progeny in the -TPO condition compared to control condition after 6 days in culture, which could indicate a delay in megakaryocytic maturation resulting in slower up-regulation of CD41.
Our results confirm the hypothesis that TPO allows for greater self-renewal of MEP, as well as the survival of both the E and Mk-destined progenitors, however TPO is not instructive in MEP lineage commitment as evidenced by no change in the frequency of colony types. These findings also support the use of TPO in cultures of less committed progenitors to promote expansion leading to higher production of transfusable cell types. Further uses of this approach can include measuring kinetics of cellular processes such as division rate and motility, which may correlate with cell state and permit predictions of the probability of lineage choices downstream of specific progenitor populations.
References:
1. Sanada and Xavier-Ferrucio et al. 2016
2. Scanlon, Kochugaeva et al. ASH. 2019
3. Fox, Priestley et al. 2002
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal